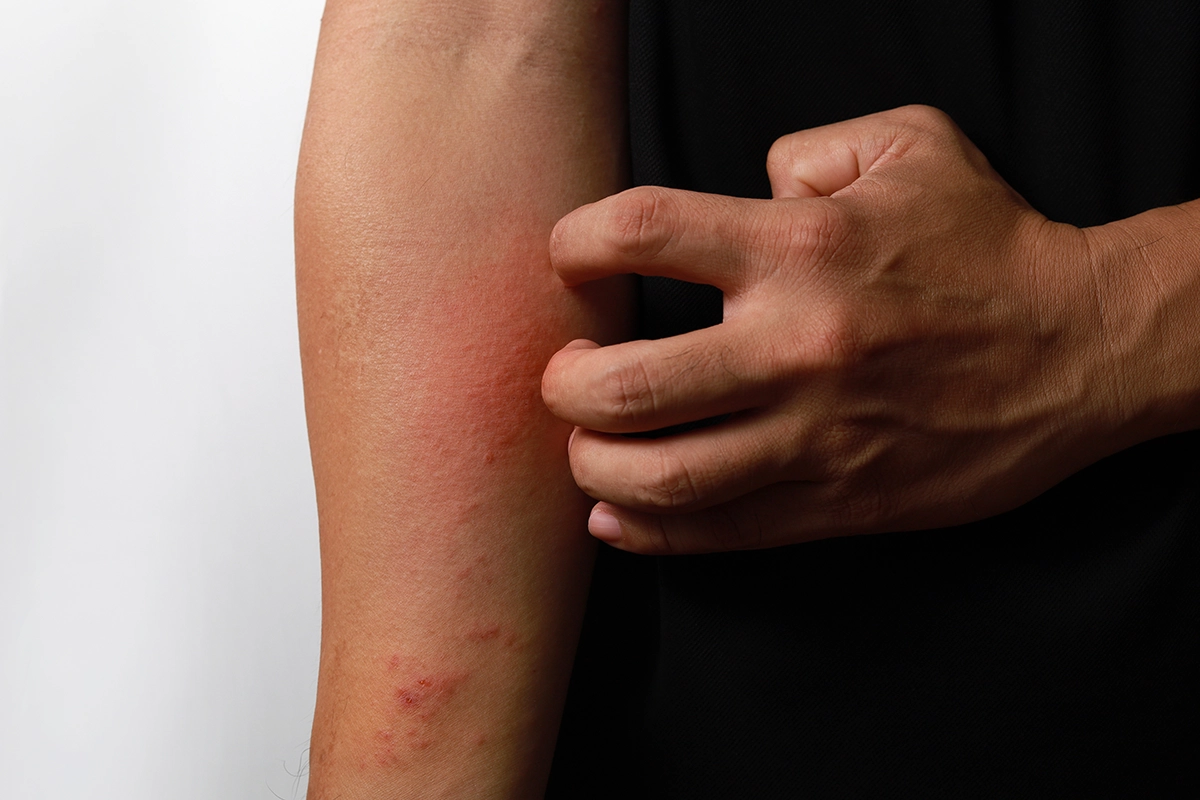
Explore Rinvoq as a Promising Option for Atopic Dermatitis Relief
Are you or a loved one aged 18-64 struggling with moderate-to-severe Atopic Dermatitis that hasn’t responded adequately to Dupixent? This SWITCH-UP clinical trial offers a unique opportunity to explore a new treatment with Rinvoq. Participants will benefit from cutting-edge care, contribute to essential research, and receive compensation for their involvement. Be a part of this transformative study aimed at advancing skin health.
Overview
Recruiting start date
Duration
Population
Individuals aged 18-64 with moderate-to-severe Atopic Dermatitis (AD) that has shown inadequate response to Dupixent.
Study Drug
Rinvoq 15 -> 30mg (50%) or Dupixent (50%) monotherapy.
Study Design
Open-label, efficacy assessor-blinded study evaluating Rinvoq initiated at 15 mg compared to Dupixent monotherapy in patients with inadequate response to dupilumab after at least 6 months of current use (last dose within 2 weeks prior to study Baseline). Patients deemed to not have an adequate response switch to Rinvoq at week 8.