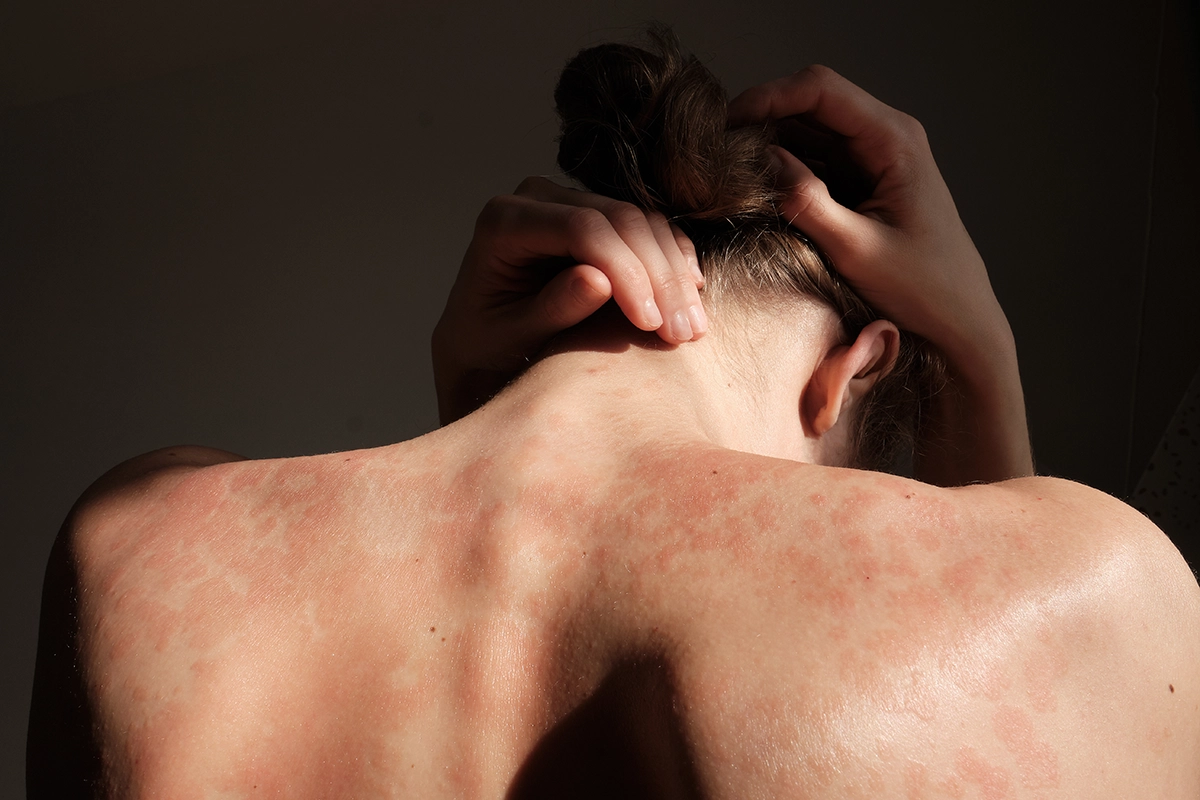
Join Us in Exploring Innovative Treatments for Plaque Psoriasis
This study evaluates the efficacy and safety of Ixekizumab alone or in combination with Tirzepatide in adults with moderate-to-severe plaque psoriasis and obesity or overweight. It is a Phase 3b, randomized, multicenter, open-label study (TOGETHER-PsO).
Overview
Recruiting start date
Duration
Study Drug
Ixekizumab / Tirzepatide
Delivery
Subcutaneous Injection
Sponsor
Eli Lilly and Company
Phase
3
Mechanism of Action
Non-radiographic axial spondyloarthritis, targets and neutralize the interleukin-17A (IL-17A) protein.
Inclusion Criteria
Exclusion Criteria
Important Notes:
The main purpose of this study is to demonstrate that when participants with moderate to severe plaque psoriasis and obesity or overweight receive Ixekizumab and Tirzepatide concomitantly administered, participants see improvement in their psoriasis and achieve weight reduction compared to when receiving Ixekizumab.
Participation in this study includes up to 12 visits and could last up to 61 weeks including screening, open-label treatment period, and post-treatment follow-up period.