Advance Hidradenitis Suppurativa Treatment Through Our NYC Study
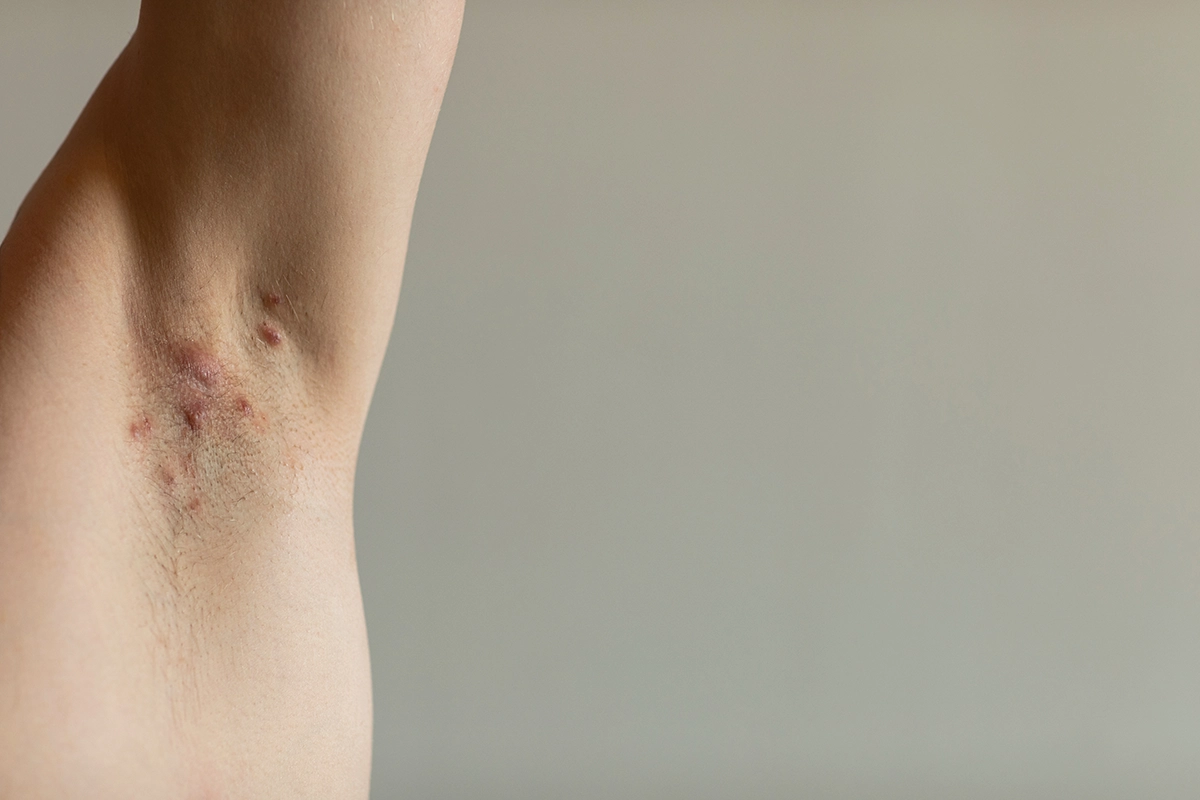
Are you or someone you know affected by moderate-to-severe Hidradenitis Suppurativa (HS)? This challenging condition, characterized by painful nodules and abscesses, significantly impacts daily life. Join our Phase 3 randomized controlled trial (RCT) in NYC to help advance treatment options specifically for HS with Remibrutinib.
We’re seeking individuals diagnosed with moderate-to-severe HS lasting at least 6 months to join our clinical trial. By participating, you’ll receive specialized care and monitoring while contributing to medical advancements focused on improving HS management.
Overview
Recruiting start date
Duration
Population
Adults with moderate-to-severe HS ≥ 6 months.
Study Drug
Remibrutinib
Details: Participants will be randomly assigned to receive either Remibrutinib (66%) or a placebo (23%). After Week 16, all participants will receive remibrutinib 10 mg b.i.d. or remibrutinib 25 mg b.i.d. up to Week 68 in a blinded way.
Design
Phase 3 RCT