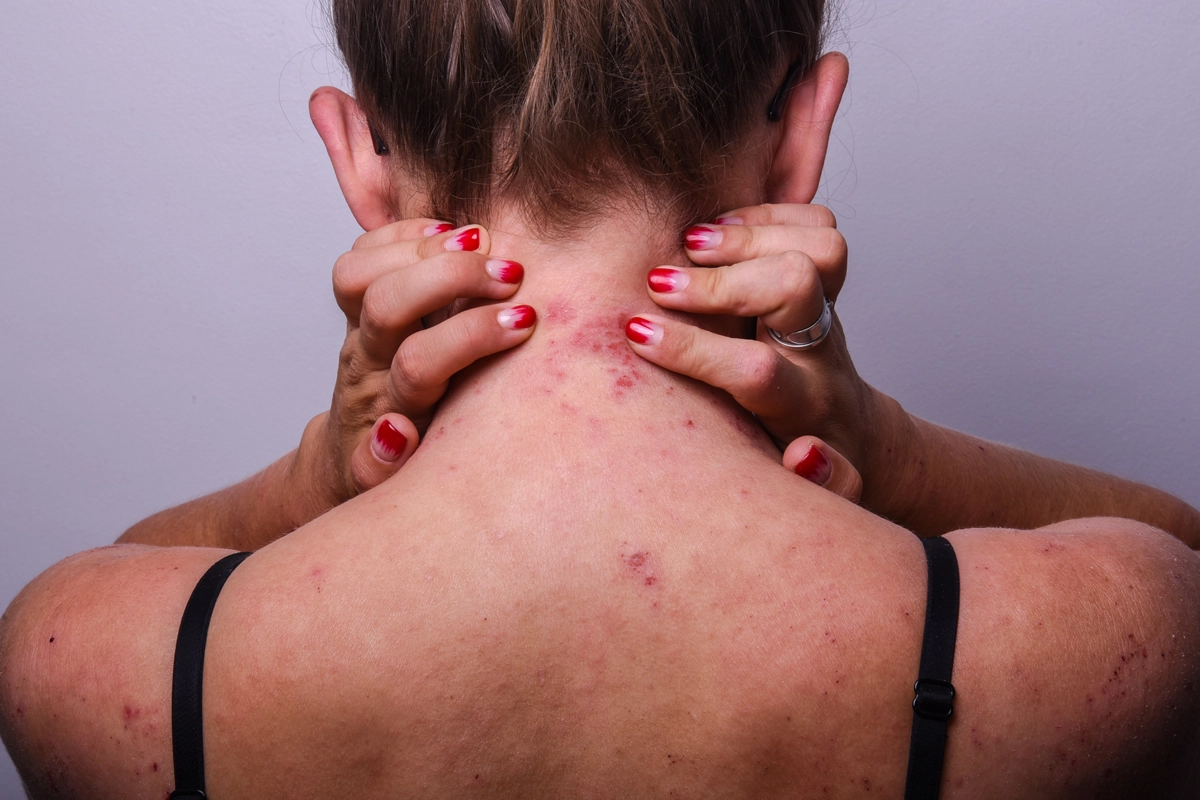
Explore the Potential of APG777 for Treating Atopic Dermatitis
Part A will evaluate the safety and efficacy of one induction dose regimen of APG777 compared to placebo. In addition, two maintenance regimens will be evaluated in Part A. Part B will evaluate the benefit-risk of 3 dose regimens of APG777 compared to placebo. One maintenance regimen will be evaluated in Part B. The study duration for any individual participant will be up to 106 weeks which includes: screening, induction, maintenance, and post-treatment follow-up periods. Participants randomized in Part A are not permitted to participate in Part B.
Overview
Recruiting start date
Duration
Population
18 Years and older (Adult, Older Adult ) With Atopic Dermatitis
Study Drug
APG777
Inclusion Criteria
Exclusion Criteria
What You Can Expect
The study will include visits to our study center where the patient will initially receive a thorough physical examination, including an EKG and blood work at no cost to the patient to assess the participants baseline health. Moreover, the patient will receive a screening examination from a dermatology specialist to confirm the diagnosis of the study disease. After informed consent and if the participants elects to continue in the study, the patient will either be given the active medication (i.e. APG777) or a placebo. There will be regular follow up visits to assess the effectiveness and safety of the therapies.