Overview
Recruiting start date
Duration
Population
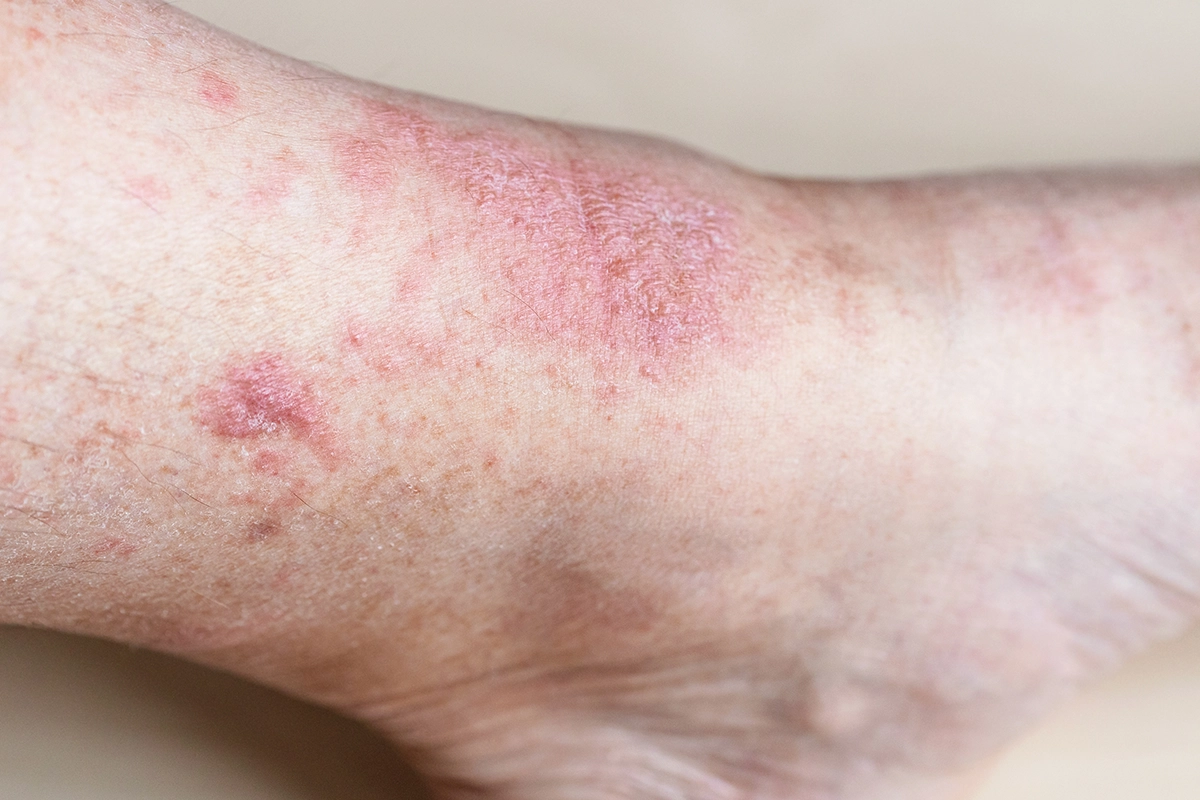
Individuals aged 12 and above with moderate-to-severe Atopic Dermatitis (AD) that is inadequately controlled with topical treatments.
Study Drug
Subcutaneous Amlitelimab injection (80%) or placebo (20%) monotherapy injection. Subjects randomized to placebo who are non-responders will receive drug after 20 weeks.
Background: Amlitelimab, an OX40 ligand inhibitor, showed best-in-class efficacy, potential disease modification, and no dose-responsive safety signals in its phase 2B trial. This study aims to further explore the efficacy and safety of Amlitelimab in a larger population.
Inclusion Criteria
Exclusion Criteria
Prior/Concomitant Therapy
Previous use of oral or topical JAK inhibitors is allowed if not exceeding 6 months. A 12-week washout period is required for biologics such as Dupilumab, Tralokinumab, and Lebrikizumab prior to the baseline visit.